According to a research report by Stratview Research, the European in vitro diagnostics market was estimated at USD 15.29 billion in 2022 and is likely to grow at a CAGR of 5.08% during 2023-2028 to reach USD 20.66 billion in 2028.
Market Dynamics
Major factors driving the growth of the European in vitro diagnostics market are-
Growing incidence of chronic and infectious illnesses: Europe has been experiencing an upswing in the prevalence of chronic health conditions, encompassing cancer, cardiovascular disorders, diabetes, and respiratory diseases. Furthermore, the emergence of infectious diseases, such as the recent COVID-19 pandemic, has underscored the imperative for precise and prompt diagnostic testing.
Based on the GLOBOCAN report of 2020, Germany recorded approximately 628,519 new cancer cases within the year. Among females, breast and colorectal cancer were found to be more prevalent, while prostate and lung cancer were more prevalent among males. In a bid to enhance the lives of over three million individuals affected by cancer by 2030, the European Union launched two initiatives in 2020. These initiatives primarily focus on raising awareness about the significance of cancer diagnosis. Consequently, with the increasing diagnosis of chronic diseases like cancer in Europe, the market for in-vitro diagnostics is poised to witness robust growth in the near future.
Shifting demographics: Europe is characterized by a notable aging population that is susceptible to a higher incidence of chronic diseases, necessitating frequent monitoring and diagnostic procedures. The expanding elderly demographic has led to an increased demand for diagnostics, crucial for the early detection and effective management of age-related conditions.
Progress in technology: Uninterrupted strides in technology have propelled significant enhancements in the precision, efficacy, and efficiency of diagnostic tests. Revolutionary methodologies, including molecular diagnostics, point-of-care testing, and automation, have broadened the horizons of diagnostic capabilities and expedited the widespread adoption of in-vitro diagnostics (IVD) tests.
Governmental support and investment: Governments across Europe have been actively investing in healthcare infrastructure while advocating for early diagnosis and preventive healthcare initiatives. Such endeavors frequently encompass funding research and development in the field of diagnostics, as well as facilitating the adoption of state-of-the-art diagnostic technologies.
Market integration and collaborative alliances: In the European IVD market, a notable trend has emerged with multiple instances of mergers, acquisitions, and collaborations among companies. These endeavors aim to fortify product portfolios, extend geographical presence, and leverage synergistic opportunities. The strategic partnerships have proven instrumental in fostering market expansion and pooling resources, expertise, and distribution networks to achieve collective growth.
What are in vitro diagnostics?
In vitro diagnostics (IVD) tests are done on samples such as blood or tissue that have been taken from the human body. These tests are used to detect diseases and infections and to monitor the health of a person to treat and prevent diseases. In vitro simply means ‘in glass, thus these tests are performed in test tubes and similar equipment.
Report Features
This report provides market intelligence in the most comprehensive way. The report structure has been kept such that it offers maximum business value. It provides critical insights into the market dynamics and will enable strategic decision-making for the existing market players as well as those willing to enter the market. The following are the key features of the report:
- Market structure: Overview, industry life cycle analysis, supply chain analysis
- Market environment analysis: Growth drivers and constraints, Porter’s five forces analysis, SWOT analysis
- Market trend and forecast analysis
- Market segment trend and forecast
- Competitive landscape and dynamics: Market share, product portfolio, product launches, etc.
- Attractive market segments and associated growth opportunities
- Emerging trends
- Strategic growth opportunities for the existing and new players
- Key success factors
Segments' Analysis
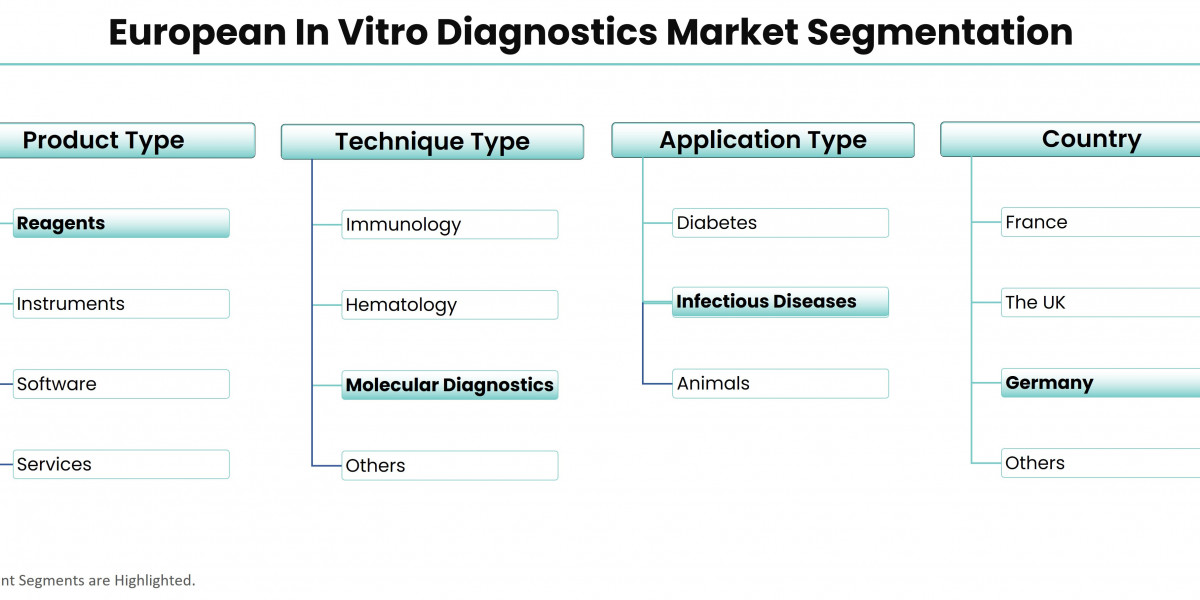
Based on the product type, the market is segmented as instruments, reagents, software, and services. The reagents segment is expected to hold the major share of the market during the forecast period owing to its high precision and accuracy in diagnosis. Furthermore, the Covid-19 pandemic has increased the demand for reagents. Also, an increase in the use of self-diagnosis tests and the number of R&D activities is expected to drive market growth during the forecast period.
Based on the technique type, the market is segmented as immunology, hematology, clinical chemistry, molecular diagnostics, coagulation, microbiology, and others. The molecular diagnostics segment is anticipated to register high growth in the market owing to the increasing demand for molecular diagnosis in infectious diseases. Furthermore, the rapid rise in chronic diseases, as well as the increase in the number of genetic disorders, is expected to drive market growth during the forecast period.
Based on the application type, the market is segmented as infectious diseases, diabetes, oncology/cancer, cardiology, nephrology, autoimmune diseases, drug testing, and others. The infectious diseases application type segment is estimated to account for the major share of the market during the forecast period on account of the increasing number of infectious diseases such as HIV-AIDS, hepatitis, and Respiratory Syncytial Virus.
In terms of country, Germany is estimated to be the leading country in the market over the forecast period. The growth of the market is owing to the increasing prevalence of infectious diseases and the growing demand for rapid tests. Furthermore, the presence of various manufacturing companies is expected to fuel market growth over the forecast period. The UK is also expected to offer substantial growth opportunities during the forecast period.
Research Methodology
This report offers high-quality insights and is the outcome of a detailed research methodology comprising extensive secondary research, rigorous primary interviews with industry stakeholders, and validation and triangulation with Stratview Research’s internal database and statistical tools.
More than 1,000 authenticated secondary sources, such as company annual reports, fact books, press releases, journals, investor presentations, white papers, patents, and articles have been leveraged to gather the data.
We conducted more than 10 detailed primary interviews with the market players across the value chain in all four regions and with industry experts to obtain both qualitative and quantitative insights.
About Us:
Stratview Research is a global market research firm that delivers precise data and ground-breaking business study, helping organizations of all sizes make suitable decisions. We gather information for our clients, assisting them to address various challenges, and trends related to their market.