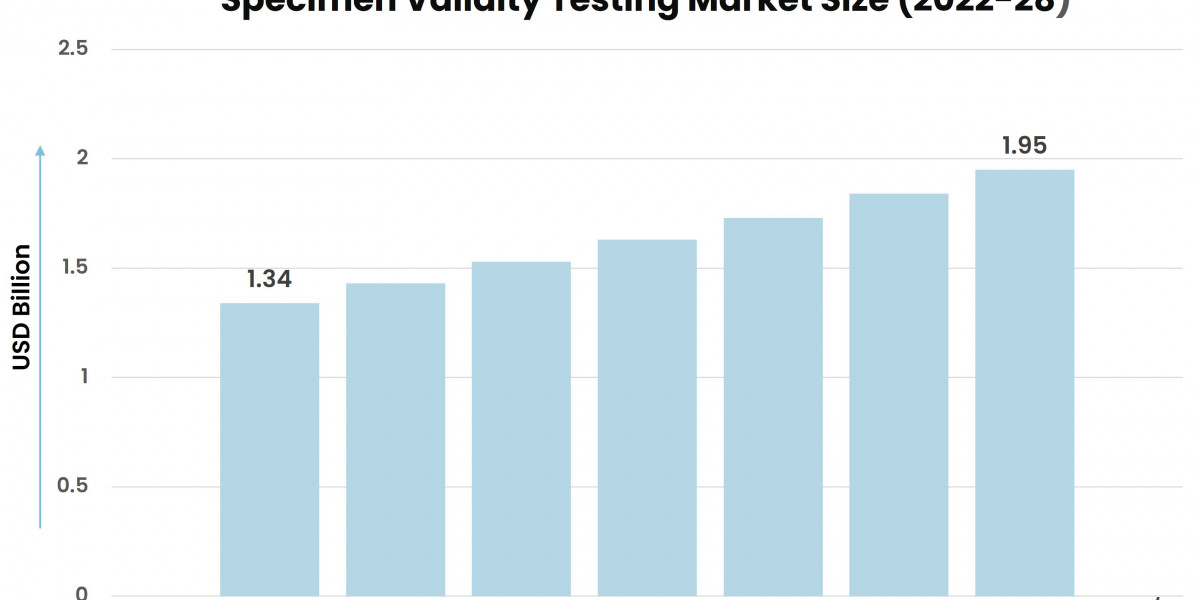
According to Stratview Research, the specimen validity testing market was estimated at USD 1.34 billion in 2022 and is likely to grow at a CAGR of 6.39% during 2023-2028 to reach USD 1.95 billion in 2028.
In the realm of healthcare and diagnostics, the Specimen Validity Testing (SVT) market stands as a silent guardian, ensuring the accuracy and integrity of clinical results. This article delves into the intricate dynamics of the SVT market, exploring its significance, evolving trends, and the role it plays in maintaining the quality and reliability of diagnostic testing.
I. The Essence of Specimen Validity Testing:
At the heart of medical diagnostics lies the need for accurate and reliable results. Specimen Validity Testing, often performed alongside routine drug testing, ensures that collected samples are genuine, unadulterated, and representative of the patient's actual condition. This vital step prevents false positives or negatives that could lead to incorrect diagnoses and treatment decisions.
II. Market Growth Drivers:
Rising Substance Abuse Concerns: As substance abuse continues to be a global health challenge, the demand for drug testing, including SVT, has seen a significant uptick. Employers, healthcare facilities, and law enforcement agencies rely on SVT to verify the authenticity of samples and maintain the credibility of drug test results.
Expanding Healthcare Industry: The growth of the healthcare industry, coupled with an increase in diagnostic testing, contributes to the expansion of the SVT market. With more tests being conducted, the need to ensure the validity of specimens becomes paramount for accurate clinical assessments.
Stringent Regulatory Standards: Regulatory bodies are placing greater emphasis on the quality and reliability of diagnostic testing. Compliance with stringent standards drives the adoption of SVT solutions, as laboratories and testing facilities strive to meet and exceed regulatory requirements.
III. Technological Advancements:
The dynamics of the SVT market are undergoing a transformation with the integration of advanced technologies. Automated systems, sophisticated analytical techniques, and the use of cutting-edge instrumentation enhance the efficiency and precision of specimen validity testing. This technological evolution not only improves testing accuracy but also streamlines laboratory workflows.
IV. Industry Challenges and Innovations:
Adulteration Techniques: As technology advances, so do the methods employed by individuals attempting to tamper with drug test results. The SVT market is challenged to stay ahead of adulteration techniques, leading to continuous innovation in detection methods and preventive measures.
Customization and Personalization: The market is witnessing a shift towards more customizable and personalized SVT solutions. Tailored approaches cater to the specific needs of different industries, ensuring that testing protocols align with the unique requirements of diverse sectors, from workplace testing to clinical diagnostics.
V. Future Outlook:
The future of the Specimen Validity Testing market holds promise, with a projected increase in demand driven by the growing awareness of the importance of accurate diagnostic results. Continuous research and development efforts will likely lead to even more sophisticated testing methodologies, further securing the reliability of specimens in various testing scenarios.
Conclusion:
Beyond the cup, beyond the routine testing procedures, the Specimen Validity Testing market plays a crucial role in upholding the integrity of diagnostic results. As technology continues to advance, and regulatory standards become more stringent, the dynamics of this market will evolve. Understanding these dynamics is key to navigating the complexities of diagnostic testing and ensuring that the healthcare industry can continue to rely on accurate and trustworthy results in its mission to improve patient outcomes. The journey "Beyond the Cup" unveils a market that safeguards the foundation of reliable diagnostics in the ever-advancing landscape of healthcare.